
Using clinical trials as example, this publication explains the difference between patient engagement (PE) and patient experience (PX), and pictures the potential business value organizations are currently missing out on. Taking notice of some current practices such as Patient Reported Experience Measures (PREMs), Real World Evidence (RWE), and Patient Focussed Product Development (PFPD), we explore directions for more patient value. Although CX and PX have fundamental differences for life science organizations, both concepts are strongly entangled across the value chain to the patient, and must be leveraged continuously.
A growing trend for Patient Engagement (PE)
In our previous publication, we introduced the management of customer (CX) and patient experience (PX) in life sciences. Along with some basic definitions, we argued that many leaders have heard about the concepts, but sometimes lack the deeper understanding to create value. The difference between patient engagement and experience management is an example of such a misunderstanding.
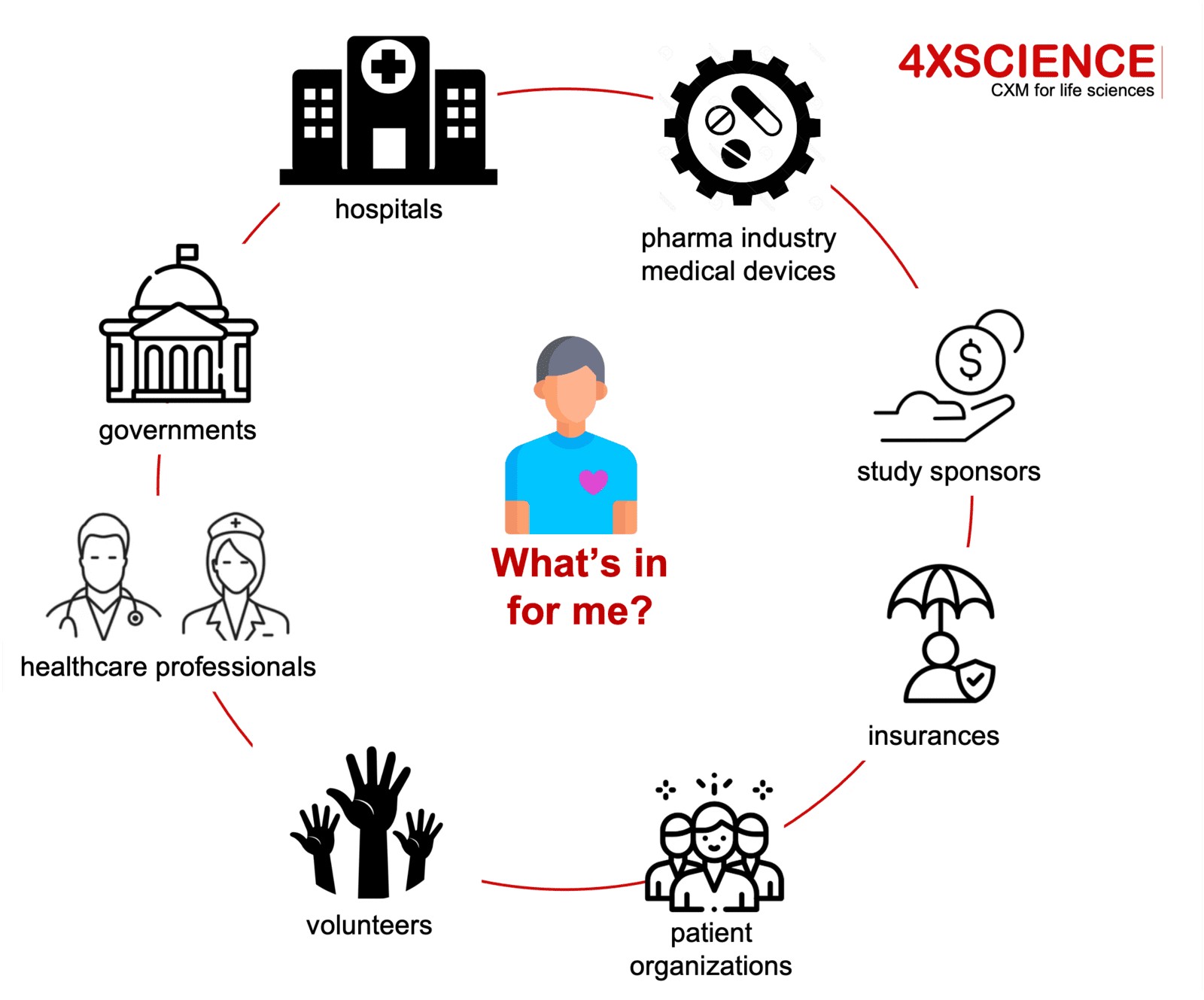
Over the last years, patients have been overwhelmed by an explosion of engagement initiatives. Not only by the industry and healthcare sector itself, but also encouraged by governments all over the world. They all want to engage with patients.
A lot of academic and professional research is published on engaging patients during clinical trial design. For example, the following EUPATI model (European Patients’ Academy on Therapeutic Innovation) shows how the industry could engage patients during development of medicinal products:
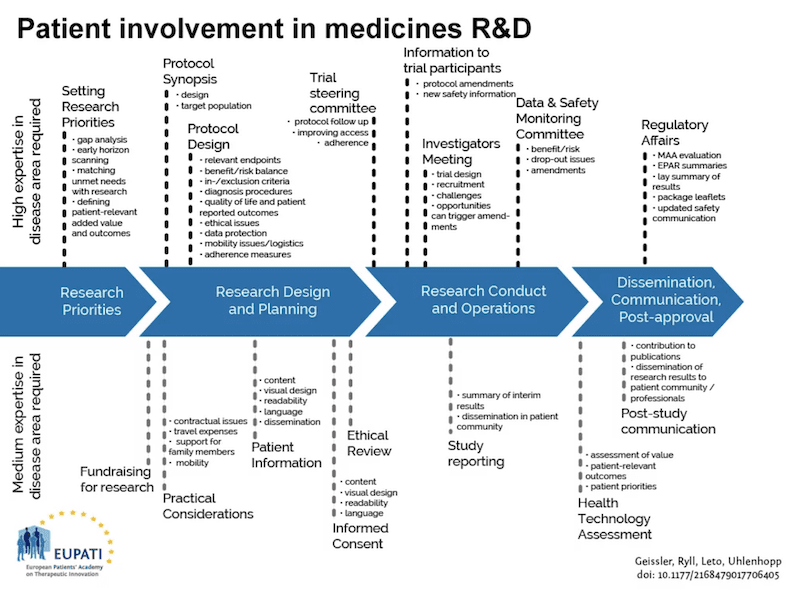
As the involvement of patients is perceived as strategic, the industry starts to develop similar models as above which identify potential points to engage patients. A direction the industry is increasingly pursuing. Patient engagement is important for all parties. In the first place for the patients themselves as it allows them to express their voice and explicitly articulate needs and expectations for specific products and services. This could then positively lead to enhanced products to the benefit of patients.
For life science organizations, early input from end consumers helps to develop better products; access to patients and organizations helps to accelerate trial recruitment; patient advocacy helps in government negotiations on reimbursement. Concepts as design thinking and customer groups are used to ensure patient input. Patient focused product development activities are drivers for both parties to further optimize interactions. Patient organizations are developing guidelines, and patients are educated to become expert in particular diseases using these structured frameworks in addition to their own disease expertise. Many non-for-profit organizations are sponsored by the industry players to get the insights from these patients transferred to their business leaders. The engagement is also enabled by regulators. Since FDA's Cures Act created a framework for evaluating the potential use of real-world evidence in support to approval of new indications, organizations are exploring options to collect and use patient real world data from a variety of sources. Real-world evidence (RWE) is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD. Real-World Data (RWD) are data relating to patient health status and the delivery of health care collected from a variety of sources. RWE can be generated by different study designs, such as randomized trials, large simple trials, pragmatic trials, and observational studies. FDA has accepted RWE to support drug product approvals, primarily in the setting of oncology and rare diseases. As a result, organizations are running hybrid trials that embed RWD from mobile devices in addition to the traditional clinical outcome formats. Benefits return back to patients: observational studies that generate innovative approaches, result in new products and treatments faster, and at a lower cost to those who need them. Thanks to patient engagement.
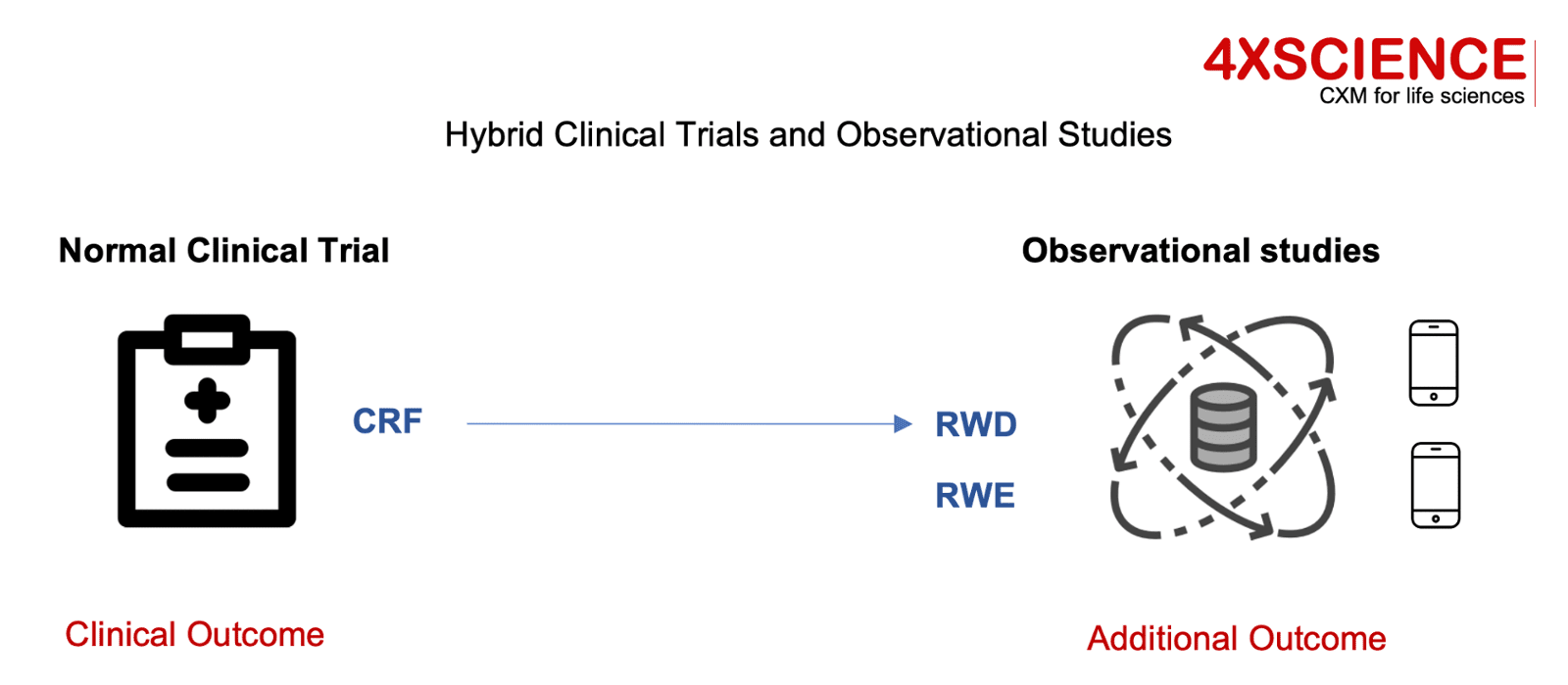
These trends confirm the strategic intent to involve patients and use their personal experiences to deliver better products and services. The basic rationale of the concept is easy: engage with patients by collaborating, listening, and involving them. Use the data that is generated in devices other than CRFs to push down on efforts required to obtain data needed for product submissions. Involve the same patients in protocol design so they feel empowered, help you with clinical trial recruiting, and give you access to their network. Later these patients might become the early adopters and help you in obtaining market appraisal from governments, customers and other patients. Bingo.
But in reality the concept is not so easy: how sustainable is the expected patient value in this patient engagement model? Not for the patient organizations, but for the patients themselves? Who gets warm from this engagement model? Some patients are motivated to save others the pain they went through; others are motivated by their role in the process, the visibility they get, the "group feeling" engagement creates, the fact of contributing to something important, belonging to a team, feeling empowered. All very valuable, but also quite soft and difficult to sustain long term. If you have been diagnosed with cancer and are seeking help from participation in clinical trials, you have other things on your mind. Imagine your child has been diagnosed: it is hard to it is hard to get excited from helping the industry with the design of a study protocol. Even if this might be a noble thing to do.
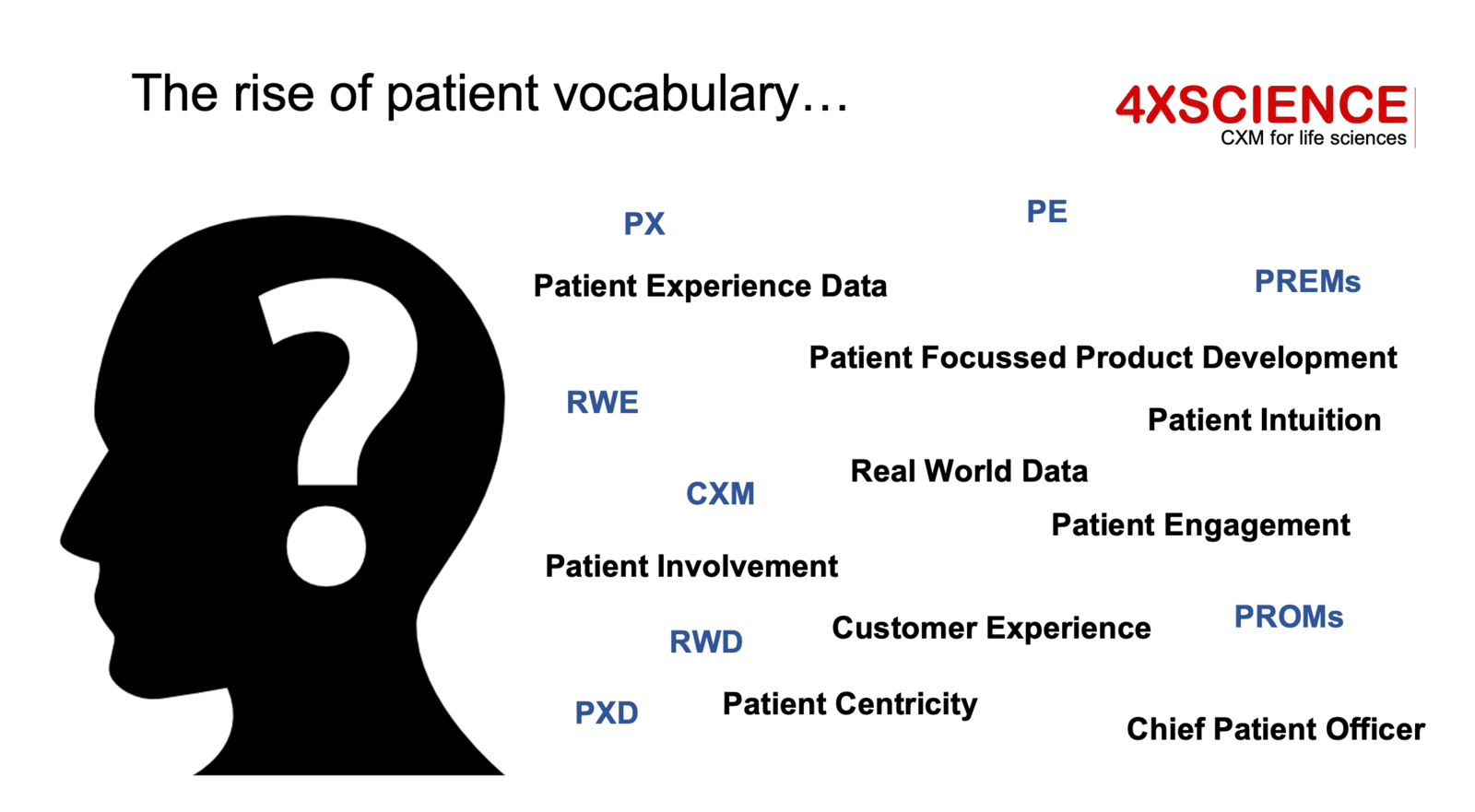
Patient engagement is certainly a positive step: a first important milestone on the path to improved PX. But it is just a step. The focus is on using experiences from patients to develop better products, and as such the main focus of the benefit is on the industry and healthcare sector itself. Along with the strong focus on engagement, we see a wild grow of new terms rising: patient engagement, patient involvement, patient intuition, patient networking, patient centricity, patient product development. As long as the word "patient" is in, it seems to be valuable... but is the value strong and sustainable enough for the patient itself, whomever that is?
Moving from PE to PX: The Patient Experience
Let's first clarify the difference between PE and PX. PX does not refer to the use of patient experiences as explained above. PE mainly draws on leveraging the "insight experiences" from patients resulting from diseases. PX refers to experiences for patients as in "emotions", "adventure", "excitement", "expectations". This is much closer to the current patient-reported experience measures (PREMs) and hospital consumer assessments of healthcare providers and systems (HCAHPS) that aim to provide a more patient-centric view of healthcare. Across the United States and Europe, governments and hospitals have been capturing these experiences for years. PREMs, for example, are a great next step towards patient experience (PX) management. These reported experiences reflect much more the Voice of the Patient, specifically on personal interactions, touchpoints, experiences. Closer to PX, but not close enough. Refer back to two pictures from our previous article.
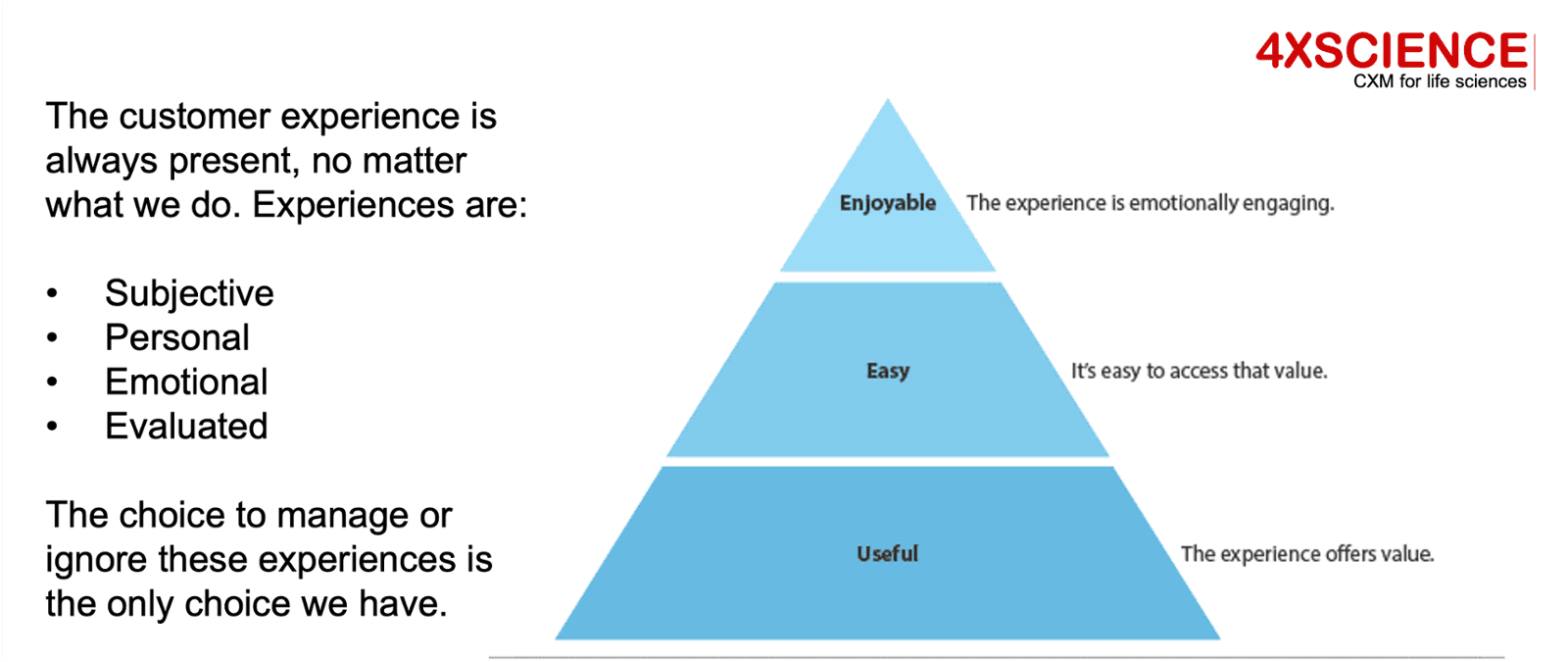
First of all, experiences should result in easy and enjoyable products and services, beyond their usefulness. Usefulness is at the basis of the model and refers to the clinical outcome: for sure this is the number one priority. The levels above have no value if the clinical outcome is below expectations. In addition, experiences are always personal and evaluated against expectations. So if we don't measure expectations, we will not know if experiences are valuable or not. PREMs is not measuring this gap, nor does it differentiate between levels. PX would address this.
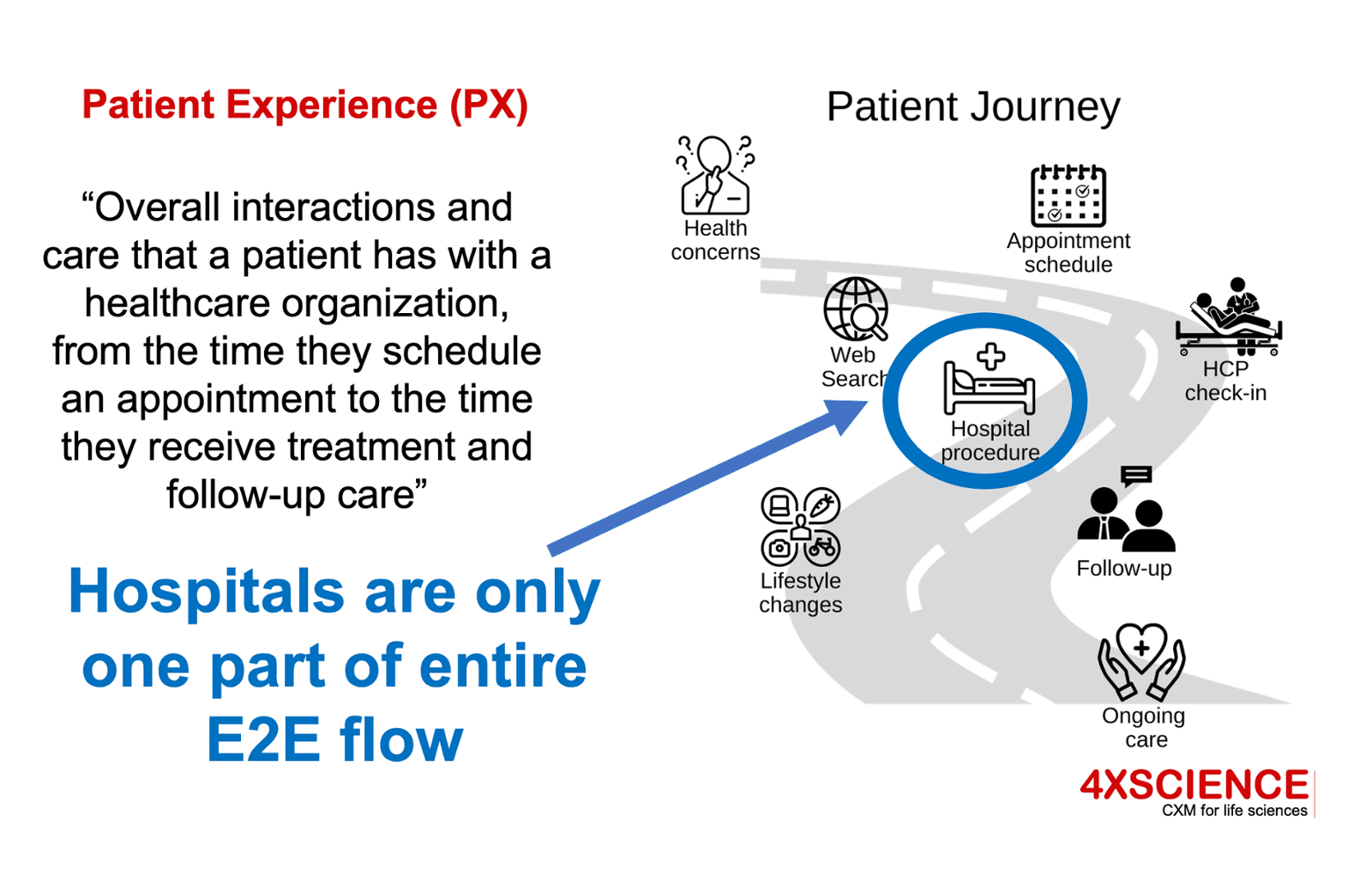
Second, is our definition of patient experience, and the patient journey reflecting all interactions and touchpoints within the end-to-end patient pathway. PREMs and HCAHPS typically stay within the walls of a hospital, but these hospitals are just one of the stakeholders in the entire process. PX should look at the end-to-end view of the patient, and evaluate the experiences versus the expectations across that spectrum.
Back to our example. Applying the PX definition to clinical trials, gives us a simplified patient journey that includes following steps from diagnosis to outcome. In this journey, the patient will continuously evaluate their own expectation versus the experiences encountered. See red line versus blue line. Some interactions will meet expectations, while others will not:
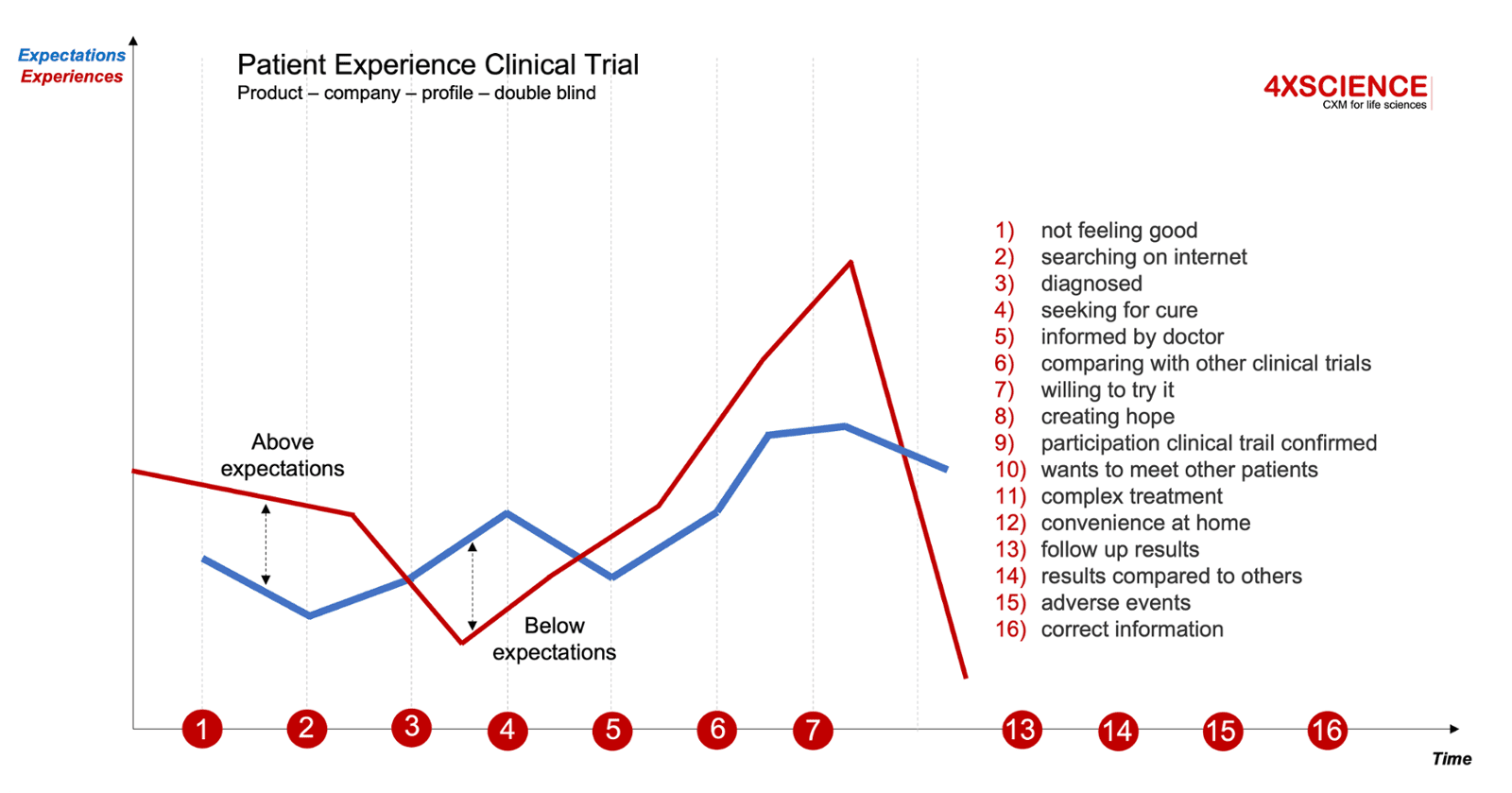
Journeys are personal: that means the journey is different for every patient. If in clinical trials phase 3, we have 10.000 patients involved, and our flow captures 50 interactions, that would results in 500.000 data points, multiplied by two for experience versus expectation. These 1M points give us an indication how we perform. If 10.000 patients evaluate us positively, that creates a significant effect to the outside world. Not only for the clinical trial itself, but for the commercial value of our product, and for the following trials from our organization. We are also setting a standard for our competitors.
There is an entire science behind implementing this. In some interactions we want to excel, in others we simply do not want to excel. Similar as we explained for CX, we need to strategically select these moments of truth that matter for our patients. And this is where we see many flaws.
Patient centricity means looking at this from a patient point of view: this is far more than involving or engaging patients. Although engagement is a positive evolution, it is not at the level of PX as we expect it. Despite strong efforts from patient organizations, delivering the benefits of the engagement to the patient itself, remains a challenge. Remember our introduction picture of a patient collaborating with the industry, with hospitals, helping patient organizations, facilitating recruitment, giving input in protocol designs, attending company events; but questioning at night "what's in for me?". Engagement will be though to sustain. The challenges to find practical benefits within a win-win model, remain.

Patient Experience: delivering strategic value
When looking beyond the clinical outcome, there are still a lot of missed opportunities between the interests from the industry and hospitals versus the benefits generated for patients. Companies will need to start paying forward, by selecting the right patient-oriented strategies. The step from Patient Engagement to Patient Experience is a clear and an easy one to make, certainly for those that have a patient first mandate as mission. But then, finding the win-wins seems to be another challenge. When life science organizations define their digital supply chain strategy, they must understand the entire end-to-end patient journey, and use these insights to select the right technologies leading to a win-win for all parties.
There are some great achievements made, for example by using apps that support the entire patient pathway, but at the same time significant opportunities still exist to turn these into real PX management. Also many new projects go beyond the PX challenge. Let's take an example of a wrong focus to clarify the statement: the informed e-consent. Life science companies are heavily investing in electronic Informed Consent (eIC) technologies. Pictured as a novel advancement to the benefit of patients. And sure there is a lot of good value for patients, but who is getting the most benefit from having the informed consent electronically versus on paper? For a patient, it does not matter to sign off on paper; in fact what a patient wants to see is more responsibilities being shifted to the manufacturer and hospital running the trial. Having the content of the text simplified, better understood. Having some options so his relatives are protected in case needed. Open, honest and clear information on all side effects. For the patient, it would be more beneficial to change the insurance text that is hard to read, and of which the main objective is to protect the company, not necessarily the customer. Although informed e-consent generates significant value for the business, it does not pass the PX level: in fact it is a red flag for PX if the content, approach, liabilities and presentation formats are not changed.
An important moment of truth for a patient is the clinical outcome. Within our same clinical trial example, this is not a guaranteed outcome. Some patients didn't have any chance at all as they were allocated placebo... what is the story we bring to them? How will they ever talk positive about the new product if we don't allow them to retake the treatment. What if they understand that the healthcare professional was aware of the placebo in a single blinded trial, months before the patient did? Issues to be solved by improved PX management. Along the same lines, we see a much stronger patient empowerment. Why would a patient participate in your clinical trial and not in the one from your competitor? Is your organization capturing the patient experience today during clinical trials? If not, how can you conclude you are doing well on PX metrics?
Management of the patient experience is important for all life science actors. It must be addressed across the entire value chain, with inclusion of all partners, and with deep understanding of the available capabilities. Once the right PX strategy is defined, it must lead in setting out the right digital supply chain strategy, and must be implemented successfully confirming patient value. There are so many visions and theories developed by the industry, by patient organizations, by hospitals, that never materialize. I remember seeing a hospital board room looking at virtual reality movies to understand the patient's viewpoint better. Fun, but it does not bring value to patients.
Let's make the step to PX and let's focus on making our patient first mission real.
In this publication, we introduced some deeper insights into PX without giving away solutions... but we have hopefully triggered some new ideas. Our next article will explain how CX and PX are used to strengthen the value. But the odds are changing. Our publications aim to create more awareness around the potential of CX and PX in life sciences. A first step in getting things done.

